

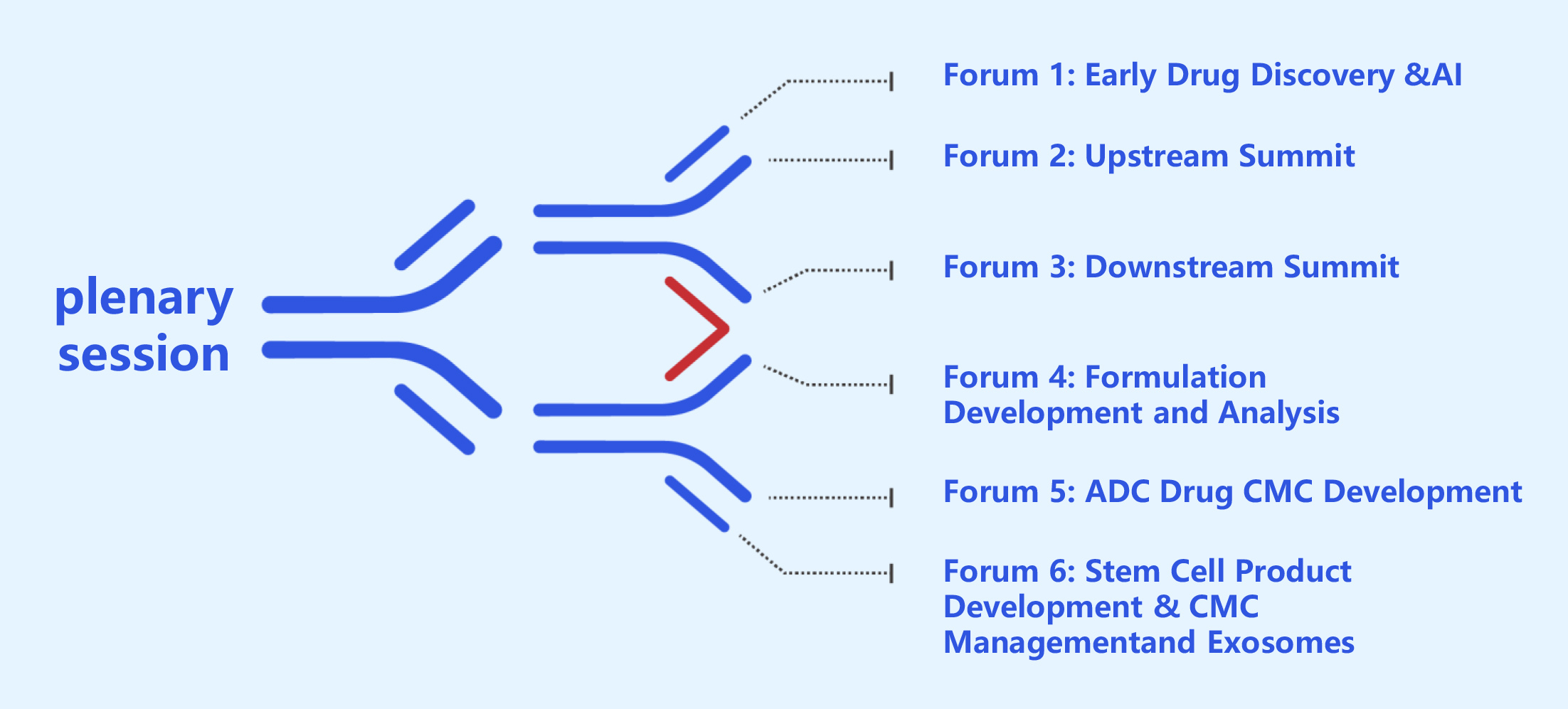
大会聚焦生物药工业化进程核心挑战,围绕A1技术应用、抗体蛋白药物(双抗新与路径。分设6大核心论坛,直击早期发现核心与趋势、上下游工艺开发与键技术、工艺放大/优化/变更/验证、生产、制剂开发与分析、质量合规等痛点、成本优化卡点,汇聚行业翘楚,紧扣当下难点热点,全力追踪最新技术,为生物药行业的蓬勃发展注入澎湃动力。
The conference focuses on the core challenges in the industrialization process of biologics, covering the application of AI technologies and antibody/protein drugs (bispecific antibodies and novel pathways). It features six core forums addressing pain points and bottlenecks such as early-stage discovery trends, upstream and downstream process development and key technologies, process scale-up/optimization/change/validation, production, formulation development and analysis, as well as quality compliance and cost optimization. Gathering industry leaders, the event closely addresses current difficulties and hot topics, fully tracking the latest technologies to inject powerful momentum into the thriving biologics industry.
会议名称丨BioCMC2025第九届百世生物药CMC技术创新大会
Conference Name | BioCMC 2025: The 9th Best Biologics CMC Technology Innovation Conference
主办单位丨百世传媒|Best Media
Organizer | Best Media
支持单位丨百世药学院、药方舟、佰仕问问
Supporting Units | Best Pharmaceutical College, Pharmacxo, Best Nect
专家顾问委员会丨徐学健、康云、史力、李秀艳、马步勇、范靖
Expert Advisory Committee | Xu Xuejian, Kang Yun, Shi Li, Li Xiuyan, Ma Buyong, Fan Jing
举办时间丨2025年9月4-5日
Date | September 4-5, 2025
举办地点丨中国 · 苏州
Location | Suzhou, China


全体大会 9月4日
Plenary Session
4th September
08:50 主办方致欢迎辞
08:50 Welcome Address by Organizer
09:00 生物技术药物质量控制研究与新版药典重组药物增修订介绍
09:00 Research on Quality Control of Biotechnology Drugs and Introduction of Revisions and Additions for Recombinant Drugs in the New Pharmacopoeia
饶春明,生检所重组药物室原主任,中国食品药品检定研究院
Chunming Rao, Former Director, Recombinant Drug Department, National Institutes for Food and Drug Control (NIFDC)
09:30 生产工艺创新案例分享
09:30 Sharing Cases of Production Process Innovation
康云,首席运营官,金赛药业
Yun Kang, Chief Operating Officer, GenSci Pharma
10:00 生物药CMC与临床开发的紧密衔接
10:00 The Close Connection Between Biologics CMC and Clinical Development
刘巨洪,总裁,全球注册顾问公司
Juhong Liu, President, Global Regulatory Consulting Firm (GRCF)
10:30 嘉宾合影 & 茶歇交流
10:30 Group Photo & Networking Tea Break
11:00 未来生物制药工艺及生产破坏性创新的契机
11:00 Opportunities for Disruptive Innovation in Future Bioprocessing and Production
李世雄,创始人、CEO,Bio GENEXUS
Shixiong Li, Founder & CEO, Bio GENEXUS
11:30 圆桌讨论:生物药出海的挑战与应对策略
11:30 Panel Discussion: Challenges and Strategies for the Global Expansion of Biologics
主持:康云,首席运营官,金赛药业
Moderator: Yun Kang, COO, GenSci Pharma
徐亚南,原全球外部创新合作中国区负责人,武田制药
Yanan Xu, Former Head of China Global External Innovation Cooperation, Takeda Pharmaceuticals
谢炘,执行董事、资深副总裁,中国生物制药
Xin Xie, Executive Director & Senior Vice President, Sino Biopharmaceutical Limited
夏明德,董事长CEO,英诺湖医药
Mingde Xia, Chairman & CEO, Innoroad Pharma
陈明久,董事长兼首席执行官,博奥信生物
Mingjiu Chen, Chairman & CEO, Biosion, Inc.
刘巨洪,总裁,全球注册顾问公司
Juhong Liu, President, Global Regulatory Consulting Firm (GRCF)
12:00 午餐交流时间
12:00 Lunch & Networking
分论坛1 药物早期发现与AI 9月4-5日
Forum 1: Early Drug Discovery & AI
4-5th September
大会主席:
Forum Chairs:
李秀艳,联席首席科学家,水木分子
Xiuyan Li, Co-Chief Scientist, Tsinghua-water.org Molecular (Shuimu Fenzi)
马步勇,教授,上海交通大学药学院
Buyong Ma, Professor, School of Pharmacy, Shanghai Jiao Tong University
13:30 多模态AI在靶点识别和验证中的应用
13:30 Application of Multimodal AI in Target Identification and Validation
任峰,CEO&CSO,英矽智能
Feng Ren, CEO & CSO, Insilico Medicine
14:15 AI驱动小分子和蛋白设计与改造的工业级平台与成功案例
14:15 Industrial-grade Platform and Success Cases of AI-driven Small Molecule and Protein Design and Engineering
潘麓蓉,董事长,圆壹智慧
Lurong Pan, Chairman, Ainnocence
14:45 从智能模型到多器官芯片:解码药企如何抢占毒性预测新蓝海
14:45 From Intelligent Models to Multi-organ Chips: Decoding How Pharma Companies Seize the New Blue Ocean of Toxicity Prediction
李秀艳,联席首席科学家,水木分子
Xiuyan Li, Co-Chief Scientist, Tsinghua-water.org Molecular (Shuimu Fenzi)
15:15 人工智能驱动肿瘤个体化精准治疗
15:15 AI-driven Personalized Precision Cancer Therapy
莫凡,联合创始人&CEO,杭州纽安津生物科技有限公司
Fan Mo, Co-founder & CEO, Hangzhou Neo-ImmuneTech Biotechnology Co., Ltd.
15:45 茶歇与交流时间
15:45 Tea Break & Networking
16:15 AI研发平台及落地实践
16:15 AI R&D Platform and Implementation Practices
鲜翾,首席信息官&数字研究院院长,金赛药业
Xuan Xian, Chief Information Officer & Dean of Digital Research Institute, GenSci Pharma
16:45 以计算医学建立生物医药产业的第一性原理:AI+疾病
16:45 Establishing the First Principles of the Biomedicine Industry with Computational Medicine: AI + Disease
赵宇,副主任,图灵-达尔文实验室
Yu Zhao, Deputy Director, Turing-Darwin Lab
17:15 圆桌谈论:AI热浪下的冷思考:药企如何制定下一阶段的 AI 战略---技术押注·战力构建·风险破局?AI制药:钱往哪儿花?人往哪儿找?药企如何布局不踩坑?
17:15 Panel Discussion: Cool Thinking Under the AI Wave: How Pharma Companies Formulate Next-stage AI Strategies—Technology Bets, Capability Building, Risk Resolution? AI Drug Discovery: Where to Spend Money? Where to Find Talent? How to Layout Without Stepping into Pitfalls?
主持:侯信成,研发负责人,Zenovabio
Moderator: Xincheng Hou, R&D Head, Zenovabio
欧阳德方,教授,澳门大学
Defang Ouyang, Professor, University of Macau
夏明德,董事长CEO,英诺湖医药
Mingde Xia, Chairman & CEO, Innoroad Pharma
李秀艳,联席首席科学家,水木分子
Xiuyan Li, Co-Chief Scientist, Tsinghua-water.org Molecular (Shuimu Fenzi)
吴思晋,助理教授,西浦慧湖药学院
Sijin Wu, Assistant Professor, XJTLU Wisdom Lake Academy of Pharmacy
鲜翾,首席信息官&数字研究院院长,金赛药业
Xuan Xian, CIO & Dean of Digital Research Institute, GenSci Pharma
18:00 第一天大会结束
18:00 Day 1 Conference Concludes
第二天
Day 2
09:00 蛋白质及复合物动态结构预测与应用
09:00 Prediction and Application of Dynamic Structures of Proteins and Complexes
马步勇,教授,上海交通大学药学院
Buyong Ma, Professor, School of Pharmacy, Shanghai Jiao Tong University
09:30 ADC药物的靶点发现和成药性---ACR246案例分析
09:30 ADC Drug Target Discovery and Druggability — ACR246 Case Analysis
苗振伟,董事长兼CEO,爱科瑞思&英百瑞
Zhenwei Miao, Chairman & CEO, Accuractis & Immunoregene
10:00 蛋白质组学如何赋能药物精准开发
10:00 How Proteomics Empowers Precision Drug Development
郑勇,董事长&首席运营官,英诺迈博
Yong Zheng, Chairman & COO, Innomab Therapeutics
10:30 茶歇与交流时间
10:30 Tea Break & Networking
11:00 AF3与结构生物学
11:00 AF3 and Structural Biology
范成鹏,教授,武汉大学
Chengpeng Fan, Professor, Wuhan University
11:30 AI赋能的大分子与小分子药物设计
11:30 AI-empowered Macromolecular and Small Molecule Drug Design
黄俊杰,副研究员,中国科学院杭州医学研究所
Junjie Huang, Associate Researcher, Hangzhou Institute of Medicine, Chinese Academy of Sciences
12:00 午餐交流时间
12:00 Lunch & Networking
13:30 非喜树碱类TOPO1抑制剂平台:解决现有ADC药物耐药的未满足临床需要
13:30 Non-Camptothecin TOPO1 Inhibitor Platform: Addressing Unmet Clinical Needs in Existing ADC Drug Resistance
党群,总裁,真实生物
Qun Dang, President, Realy Biotechnology
14:00 AI赋能,多靶点双功能payload解决ADC耐药
14:00 AI-empowered, Multi-target Bifunctional Payload to Solve ADC Resistance
张龙,联合创始人,德睿智药
Long Zhang, Co-founder, MindRank AI
14:30 茶歇与交流时间
14:30 Tea Break & Networking
15:00 AI赋能培养基组分的优化
15:00 AI-empowered Optimization of Culture Medium Components
宋春雨,总经理,浙江壹生科生物技术有限公司
Chunyu Song, General Manager, Zhejiang Yishengke Biotechnology Co., Ltd.
15:30 AI赋能抗体早研和工艺开发
15:30 AI-empowered Antibody Early Research and Process Development
齐凯,CTO,大湾生物
Kai Qi, CTO, Great Bay Bio
16:00 圆桌议题:蛋白质组学、蛋白质动态结构预测在 ADC 药物耐药机制研究与设计优化中的作用,及生物药企业技术转化与临床应用挑战应对?
16:00 Panel Discussion: The Role of Proteomics and Protein Dynamic Structure Prediction in ADC Drug Resistance Mechanism Research and Design Optimization, and the Challenges in Technology Transfer and Clinical Application for Biologics Companies?
蛋白质组学技术在 ADC 药物耐药机制研究中能发挥哪些独特作用?
What unique roles can proteomics technology play in ADC drug resistance mechanism research?
蛋白质及复合物动态结构预测又能为ADC药物的 payload 设计和抗体 - 靶点结合优化提供哪些支持?
How can dynamic structure prediction of proteins and complexes support payload design and antibody-target binding optimization for ADC drugs?
在解决 ADC 药物耐药问题上,生物药企业在技术转化和临床应用过程中可能会面临哪些挑战,该如何应对?
What challenges might biologics companies face in technology transfer and clinical application processes when addressing ADC drug resistance issues, and how should they respond?
主持:党群,总裁,真实生物
Moderator: Qun Dang, President, Realy Biotechnology
马步勇,教授,上海交通大学药学院
Buyong Ma, Professor, School of Pharmacy, Shanghai Jiao Tong University
刘能银,早期药物开发部高级副总裁,派格生物
Nengyin Liu, Senior Vice President, Early Drug Development, PegBio Co., Ltd.
郑勇,董事长&首席运营官,英诺迈博
Yong Zheng, Chairman & COO, Innomab Therapeutics
齐凯,CTO,大湾生物
Kai Qi, CTO, Great Bay Bio
16:45 大会结束致辞
16:45 Closing Remarks
分论坛2 上游峰会 9月4-5日
Forum 2: Upstream Summit
4-5th September
大会主席:
Forum Chair
Xiaoshan Wang, Vice President, Yuanben Biotech (Zhuhai) Group
王晓山,副总裁,元本生物(珠海)集团
13:30 重组蛋白药物上游工艺开发
13:30 Upstream Process Development for Recombinant Protein Drugs
Xiaoshan Wang, Vice President, Yuanben Biotech (Zhuhai) Group
王晓山,副总裁,元本生物(珠海)集团
14:15 不同国家细胞库检测差异分析
14:15 Analysis of Cell Bank Testing Differences Across Countries
Hongcheng Li, Executive Deputy General Manager, Changchun Yunxi Biology
李洪成,常务副总经理,长春云熙生物
14:45 如何平衡生物药生产中的合规和效率的需求---数字化技术应用考量
14:45 Balancing Compliance and Efficiency Needs in Biologics Production - Considerations for Digital Technology Application
Ruijun Shi, CEO, Wangwang Technology
石瑞君, CEO,网网科技
15:15 利用赢创细胞培养成分高效提升与简化生物过程
15:15 Efficiently Enhance and Simplify Bioprocesses with Evonik Cell Culture Ingredients
Spandan Mishra, Global Head of Cell Culture Solutions, Evonik
Spandan Mishra,细胞培养解决方案全球负责人,赢创
15:45 茶歇与交流时间
15:45 Tea Break & Networking
16:15 双/多特异性抗体上游工艺开发及关键质量控制策略
16:15 Upstream Process Development and Key Quality Control Strategies for Bispecific/Multispecific Antibodies
Jianliang Li, Chairman, TaeGen Biologics
李建良,董事长,泰槿生物
16:45 双抗细胞株构建难点解析
16:45 Analysis of Difficulties in Bispecific Antibody Cell Line Construction
Yingchun Li, Macromolecular CMC Director, Chia Tai Tianqing Pharmaceutical Group (CTTQ)
李盈淳,大分子CMC总监,正大天晴
17:15 圆桌讨论:如何在双特异性抗体的开发与生产过程中,通过数字化技术,构建一个兼具全球合规性、生产高效性和成本效益的智能化细胞库管理体系?
17:15 Panel Discussion: How to Build an Intelligent Cell Bank Management System with Global Compliance, Production Efficiency, and Cost-effectiveness Through Digital Technology in the Development and Production Process of Bispecific Antibodies?
Moderator: Ruijun Shi, CEO, Wangwang Technology
主持:石瑞君,CEO,网网科技
Jianliang Li, Chairman, TaeGen Biologics
李建良,董事长,泰槿生物
Yingchun Li, Macromolecular CMC Director, CTTQ
李盈淳,大分子CMC总监,正大天晴
Hongcheng Li, Deputy General Manager, Changchun Yunxi Biology
李洪成,副总经理,长春云熙生物
18:00 第一天大会结束
18:00 Day 1 Conference Concludes
Day 2
第二天
09:00 细胞培养工艺案例分享-发挥CMC平台综合解决问题的能力
09:00 Cell Culture Process Case Sharing - Leveraging the Comprehensive Problem-Solving Capability of the CMC Platform
Lin Zhu, VP-CMC, Former Zelgen Biopharmaceuticals
朱琳,VP-CMC,原泽璟生物
09:45 抗体药物培养基成分选择与配方优化
09:45 Selection and Formulation Optimization of Culture Medium Components for Antibody Drugs
Fengbin Jia, Senior Director of R&D and Application, OPUM Biotechnology
贾丰彬,研发与应用部高级总监,奥浦迈生物
10:30 茶歇与交流时间
10:30 Tea Break & Networking
11:00 TCR重组蛋白药物CMC考量
11:00 CMC Considerations for TCR Recombinant Protein Drugs
Songkai Liu, Senior Vice President of Business Development, HtBio (Hangzhou) Co., Ltd.
刘嵩凯,业务拓展高级副总裁,汉腾生物
11:30 抗体药物上游工艺的智能驱动优化
11:30 Intelligent-driven Optimization of Upstream Processes for Antibody Drugs
Tuo Fu, Director of Drug Substance Process Technology, GenSci Pharma
付托,原液工艺技术总监,金赛药业
12:00 午餐交流时间
12:00 Lunch & Networking
13:30 上游工艺控制策略探讨
13:30 Discussion on Upstream Process Control Strategies
Xiande Su, Technical Lead, CTTQ Nanjing Shunxin Co., Ltd.
苏贤德,技术负责人,正大天晴南京顺欣公司
14:00 载氧体人造血、急性心梗脑梗急救药的创新研发和产业化
14:00 Innovative R&D and Industrialization of Oxygen Carrier Artificial Blood and First-aid Drugs for Acute Myocardial Infarction and Cerebral Infarction
Ziyuan Wang, Chairman, Beijing Hejing Biology
王子元,董事长,北京荷晶生物
14:30 茶歇与交流时间
14:30 Tea Break & Networking
15:00 抗体药物上游工艺表征与工艺验证的关键点与常见误区
15:00 Key Points and Common Misunderstandings in Process Characterization and Process Validation for Antibody Drug Upstream Processes
Zhiwei Pan, VP CMC, TOT Biopharm Co., Ltd.
潘志卫,CMC副总裁,东曜药业
15:30 圆桌讨论:CMC平台如何支持从"急救场景"到"慢性疾病"的极端需求药物开发?
15:30 Panel Discussion: How Can the CMC Platform Support Drug Development from "Emergency Scenarios" to "Chronic Diseases" with Extreme Requirements?
Moderator: Lin Zhu, VP-CMC, Former Zelgen Biopharmaceuticals
主持:朱琳,VP-CMC,原泽璟生物
Zhiwei Pan, VP CMC, TOT Biopharm
潘志卫,CMC副总裁,东曜药业
Songkai Liu, SVP Business Development, HtBio
刘嵩凯,业务拓展高级副总裁,汉腾生物
Ziyuan Wang, Chairman, Beijing Hejing Biology
王子元,董事长,北京荷晶生物
16:15 大会结束致辞
16:15 Closing Remarks
分论坛3 下游峰会 9月4-5日
Forum 3: Downstream Summit
4-5th September
大会主席:
Forum Chairs
Shixiong Li, Founder & CEO, Bio GENEXUS
李世雄,创始人、CEO,Bio GENEXUS
Jianhua Su, Chief Manufacturing Officer, ZhiXiang Biotech
苏建华,首席制造官,智享生物
13:30 生物药生产质量体系的构建与管理
13:30 Construction and Management of Biologics Production Quality System
Zhengkun Zhang, Global Quality System Head, Hengrui Pharma
张正坤,全球质量体系负责人,恒瑞医药
14:15 抗体填料国产替代突围之路-纳微抗体纯化解决方案
14:15 Breaking Through in Domestic Alternative of Antibody Chromatography Resins - NanoMicro Antibody Purification Solutions
Baisheng Jin, Macromolecular Application Director, NanoMicro Technology
金百胜,大分子应用总监,纳微生物
14:45 抗体纯化中宿主细胞 DNA 与蛋白残留的去除
14:45 Removal of Host Cell DNA and Protein Residues in Antibody Purification
Yilun Wang, Senior CMC Director, AiMu Therapeutics
王逸伦,CMC高级总监,蔼睦医疗
15:15 克服ADC 药物放大生产和工艺验证中的挑战
15:15 Overcoming Challenges in Scale-up Production and Process Validation of ADC Drugs
Hongbin Song, Senior Director of ADC Production Technology, Innovent Biologics
宋洪彬,ADC生产技术高级总监,信达集团
15:45 茶歇与交流时间
15:45 Tea Break & Networking
16:15 大分子生物药COG模型-从理论到实践
16:15 COG Model for Macromolecular Biologics - From Theory to Practice
Min Zhu, Senior Vice President & CTO, Zhejiang Zencro Biotech Co., Ltd. (Zencro)
朱敏,CTO高级副总裁,臻格生物
16:45 基于膜分离技术的病毒捕获与清除
16:45 Virus Capture and Clearance Based on Membrane Separation Technology
Rong Fan, PhD Supervisor, Institute of Process Engineering, Chinese Academy of Sciences
樊荣,博导,中国科学院过程工程研究所
17:15 圆桌讨论:面对各种抗体新药未来蛋白质纯代工艺如何因应?
17:15 Panel Discussion: How Should Future Protein Purification Processes Adapt to Various New Antibody Drugs?
How to ensure effective removal of new impurities?
如何确保新的杂质有效去除?
Can the quality of domestic chromatography media completely replace foreign dependence?
国产层析介质的质量能否完全取代对外国的依赖?
Can the development of filtration membrane technology enable continuous purification processes?
过滤膜技术的开发能否赋能连续性纯化工艺?
How to effectively use AI to improve downstream purification efficiency and analysis?
如何有效运用AI增进下游纯化效率及分析?
Moderator: Shixiong Li, Founder & CEO, Bio GENEXUS
主持:李世雄,创始人、CEO,Bio GENEXUS
Jianxin Chen, President & CEO, Zencro Biotech
陈建新,总裁兼首席执行官,臻格生物
Yilun Wang, Senior CMC Director, AiMu Therapeutics
王逸伦,CMC高级总监,蔼睦医疗
Rong Fan, PhD Supervisor, IPE, CAS
樊荣,博导,中国科学院过程工程研究所
18:00 第一天大会结束
18:00 Day 1 Conference Concludes
Day 2
第二天
09:00 美国 FDA拼图的撕裂与重置,2025年的进行式
09:00 The Tearing and Resetting of the US FDA Puzzle, the 2025 Progress
Shude Li, Chief Manufacturing Officer, China Resources Biotech
李树德,首席制造官,华润生物
09:45 新型免疫治疗药物双靶融合蛋白平台
09:45 Novel Bispecific Fusion Protein Platform for Immunotherapy
Jian Xu, CEO, Chengdian Biotech
徐健,CEO,乘典生物
10:30 茶歇与交流时间
10:30 Tea Break & Networking
11:00 抗体药物杂质分析与去除
11:00 Analysis and Removal of Impurities in Antibody Drugs
Rongxiu Li, Professor, Shanghai Jiao Tong University
李荣秀,教授,上海交通大学
11:30 AI在药物审评和开发中的应用
11:30 Application of AI in Drug Review and Development
Yang Gao, ISPE China Committee Member and Chair of Gene & Cell Therapy Committee; Founder & CEO, Beijing Fange Technology Co., Ltd.
高杨,ISPE中国区理事和基因细胞委员会主席;创始人&CEO,北京凡歌科技有限公司
12:00 午餐交流时间
12:00 Lunch & Networking
13:30 生物药分段生产实践探索
13:30 Exploration of Segmented Production Practices for Biologics
Juping Shen, Senior Vice President, CANbridge Pharmaceuticals
沈菊平,高级副总裁,北海康成
14:00 Q膜层析在病毒清除及生产降本增效的卓越表现
14:00 Excellent Performance of Q Membrane Chromatography in Virus Clearance and Production Cost Reduction and Efficiency Increase
Yan Yang, Director, GenSci Pharma
阳燕,总监,金赛药业
14:30 茶歇与交流时间
14:30 Tea Break & Networking
15:00 抗体商业化生产的优化方案
15:00 Optimization Solutions for Commercial Antibody Production
Jianhua Su, Chief Manufacturing Officer, ZhiXiang Biotech
苏建华,首席制造官,智享生物
15:30 圆桌讨论:生物药商业化生产全周期管理:从产能规划、成本优化到上市后变更的策略与平衡
15:30 Panel Discussion: Lifecycle Management of Commercial Biologics Production: Strategies and Balance from Capacity Planning, Cost Optimization to Post-approval Changes
Moderator: Ding Liu, Deputy General Manager, Qilu Pharmaceutical
主持:刘丁,副总经理,齐鲁制药
Shude Li, Chief Manufacturing Officer, China Resources Biotech
李树德,首席制造官,华润生物
Juping Shen, SVP, CANbridge Pharmaceuticals
沈菊平,高级副总裁,北海康成
Jianhua Su, Chief Manufacturing Officer, ZhiXiang Biotech
苏建华,首席制造官,智享生物
16:15 大会结束致辞
16:15 Closing Remarks
分论坛4 制剂与分析 9月4-5日
Forum 4: Formulation & Analysis
4-5th September
大会主席:
Forum Chair
Li Shi, Chairman, EDO Biotech
史力,董事长,怡道生物
13:30 生物医药制剂开发:艺术和生物医药的灵魂
13:30 Biopharmaceutical Formulation Development: The Art and Soul of Biopharmaceuticals
Li Shi, Chairman, EDO Biotech
史力,董事长,怡道生物
14:15 注射生物制剂中赋形剂的直接定量by NMR
14:15 Direct Quantification of Excipients in Injectable Biologics by NMR
Huiwen Deng, Pharmaceutical Market Development & Sales Manager, Bruker
邓惠文,制药市场拓展与销售经理,布鲁克
14:45 制剂的靶向技术与高效递送
14:45 Targeted Technology and Efficient Delivery of Formulations
Xun Liu, CEO, TAI-CHU Group
刘洵,CEO,泰楚集团
15:15 光散射技术在生物药领域应用
15:15 Application of Light Scattering Technology in the Field of Biologics
Hui Ning, Product Director, 丹东百特仪器有限公司 Bettersize Instruments Ltd.
宁辉,产品总监,丹东百特仪器有限公司
15:45 茶歇与交流时间
15:45 Tea Break & Networking
16:15 医用弹性体密封件的相容性研究
16:15 Compatibility Study of Medical Elastomer Seals
Zhenxia Du, Professor, Beijing University of Chemical Technology
杜振霞,教授,北京化工大学
16:45 高浓度抗体制剂:开发挑战和创新解决方案
16:45 High-Concentration Antibody Formulations: Development Challenges and Innovative Solutions
Jinshu Qiu, Vice President, Ausa Pharmed (Hengrui)
仇金树,副总裁,澳斯康生物
17:15 圆桌讨论:从实验室到市场:跨越生物药制剂与生产的核心挑战
17:15 Panel Discussion: From Lab to Market: Overcoming Core Challenges in Biologics Formulation and Production
Moderator: Mingyang Hei, Director of Formulation Process, Innovent Biologics
主持:黑明阳,制剂工艺总监,信达生物
Jiaqiang Cai, Co-founder/CSO, YL-Biopharma
蔡家强,联合创始人/CSO,宜联生物
Xun Liu, CEO, TAI-CHU Group
刘洵,CEO,泰楚集团
Jinshu Qiu, VP, Ausa Pharmed
仇金树,副总裁,澳斯康生物
18:00 第一天大会结束
18:00 Day 1 Conference Concludes
Day 2
第二天
09:00 人工智能在生物大分子制剂中的机遇与挑战
09:00 Opportunities and Challenges of Artificial Intelligence in Biologics Formulation
Defang Ouyang, Professor, University of Macau
欧阳德方,教授,澳门大学
09:45 药械组合产品FDA申报策略及案例分享
09:45 FDA Submission Strategies and Case Sharing for Drug-Device Combination Products
Shuying Ji, Head of Drug-Device Combination Product Development, WuXi Biologics
姬淑英,药械组合产品开发负责人,药明生物
10:30 茶歇与交流时间
10:30 Tea Break & Networking
11:00 抗体类药物使用中稳定性研究及稀释体系优化
11:00 Study on In-use Stability and Dilution System Optimization for Antibody Drugs
Hao Wu, Associate Professor, Shenyang Pharmaceutical University
吴昊,副教授,沈阳药科大学
11:30 预充针的生产工艺难点探讨
11:30 Discussion on Difficulties in Pre-filled Syringe Production Process
Qing Huang, Deputy General Manager, CTTQ Nanjing Shunxin Pharmaceutical Co., Ltd.
黄庆,副总经理,正大天晴药业集团南京顺欣制药有限公司
12:00 午餐交流时间
12:00 Lunch & Networking
13:30 冻干工艺开发与优化
13:30 Lyophilization Process Development and Optimization
Xiaoshan Wang, Vice President, Yuanben Biotech (Zhuhai) Group
王晓山,副总裁,元本生物(珠海)集团
14:00 蛋白类生物药制剂和质量研究若干进展
14:00 Several Advances in Formulation and Quality Research of Protein-based Biologics
Weijie Fang, Researcher, College of Pharmaceutical Sciences, Zhejiang University
方伟杰,研究员,浙江大学药学院
14:30 茶歇与交流时间
14:30 Tea Break & Networking
15:00 圆桌讨论
15:00 Panel Discussion
Xinxin Li, Senior Director of Analytics & Formulation, Simcere Pharmaceutical
李鑫鑫,分析制剂高级总监,先声药业
Hao Wu, Associate Professor, SPU
吴昊,副教授,沈阳药科大学
Xiaoshan Wang, VP, Yuanben Biotech
王晓山,副总裁,元本生物(珠海)集团
Weijie Fang, Researcher, ZJU
方伟杰,研究员,浙江大学药学院
15:45 大会结束致辞
15:45 Closing Remarks
分论坛5 ADC药物CMC开发 9月4-5日
Forum 5: ADC Drug CMC Development
4-5th September
大会主席:
Forum Chairs
Zhongli Zhang, Former General Manager of Analytical Science & Development, Henlius
张仲理,原分析科学及开发总经理,复宏汉霖
Karen Twu, VP Biologics CMC, HUTCHMED
和黄医药,生物药CMC副总裁,Karen Twu
13:30 ADC CMC 开发及案例分享
13:30 ADC CMC Development and Case Sharing
Karen Twu, VP Biologics CMC, HUTCHMED
Karen Twu,生物药CMC副总裁,和黄医药
14:15 去动物化的药物成药性研究可行性探讨及解决策略
14:15 Feasibility Discussion and Solution Strategies for Animal-free Drug Druggability Studies
Li Liu, PhD Supervisor / Co-founder, Formerly Zhijiankang (Yuanzhijian)
刘莉,博导/联合创始人,原知见康
14:45 双抗ADC质量研究与控制
14:45 Quality Research and Control of Bispecific ADC
Xujia Wang, Senior Director, Junshi Biosciences
王绪甲,高级总监,君实生物
15:15 ADC药物质量分析的难点探讨
15:15 Discussion on Difficulties in ADC Drug Quality Analysis
Linlin Wang, Director of Analytical Science Department, Henlius
王林林,分析科学部总监,复宏汉霖
15:45 茶歇与交流时间
15:45 Tea Break & Networking
16:15 FDA简史
16:15 A Brief History of the FDA
Qi Zhang, Founder, WarmChestnut Bio (Wenli Bio)
张骐,创始人,温栗生物
16:45 ADC药物的关键质量属性研究和分析
16:45 Research and Analysis of Key Quality Attributes for ADC Drugs
Xuefei Yin, Head of Mass Spectrometry Center, Asymchem Laboratories
殷薛飞,质谱中心负责人,凯莱英
17:15 圆桌讨论: 从动物模型到数字化工具:ADC成药性评价的范式变革如何推动CMC闭环?
17:15 Panel Discussion: From Animal Models to Digital Tools: How Does the Paradigm Shift in ADC Druggability Evaluation Promote CMC Closure?
Moderator: Zhongli Zhang, Former GM Analytical Science & Development, Henlius
主持:张仲理,原分析科学及开发总经理,复宏汉霖
Karen Twu, VP Biologics CMC, HUTCHMED
Karen Twu,生物药CMC副总裁,和黄医药
Hongbin Song, Senior Director ADC Production Technology, Innovent Biologics
宋洪彬,ADC生产技术高级总监,信达集团
Li Liu, Co-founder, Formerly Zhijiankang (Yuanzhijian)
刘莉,联合创始人,原知见康
Qi Zhang, Founder, WarmChestnut Bio (Wenli Bio)
张骐,创始人,温栗生物
18:00 第一天大会结束
18:00 Day 1 Conference Concludes
Day 2
第二天
09:00 ADC药物质量标准制定的难点与策略
09:00 Difficulties and Strategies in Setting ADC Drug Quality Standards
Xinfang Li, CEO, Mabplex International
李新芳,CEO,迈百瑞
09:45 不同阶段ADC药物工艺难点及调整策略
09:45 Process Difficulties and Adjustment Strategies for ADC Drugs at Different Stages
Shuze Xu, Director of Downstream & ADC Process Development, Jixing (Hangzhou) Biologics Co., Ltd. (Jixing Biologics)
徐淑泽,下游及 ADC 工艺开发总监,奕安济世
10:30 茶歇与交流时间
10:30 Tea Break & Networking
11:00 临床后期ADC工艺优化与质量控制策略
11:00 Late-stage Clinical ADC Process Optimization and Quality Control Strategy
Yuejun Xie, VP CMC Product Development, ACROBiosystems
谢岳峻,CMC 产品开发副总裁,百普赛斯
11:30 统计在药物开发与生产的可比性研究中的应用
11:30 Application of Statistics in Comparability Studies for Drug Development and Production
Shengnan Cai, CMC Statistics Lead, BeiGene
蔡圣楠,CMC统计负责人,百济神州
12:00 午餐交流时间
12:00 Lunch & Networking
13:30 ADC药物代谢生成Payload相关物质的难点、案例和策略
13:30 Difficulties, Cases, and Strategies for Metabolite-generated Payload-related Substances in ADC Drugs
Xingxing Diao, Researcher, Shanghai Institute of Materia Medica, Chinese Academy of Sciences
刁星星,研究员,中国科学院上海药物研究所
14:00 ADC生产工艺概述与关键工艺控制
14:00 Overview of ADC Production Process and Key Process Control
Yun Zhou, Director of Conjugation & Formulation Production, TOT Biopharm
周昀,偶联及制剂生产总监,东曜药业
14:30 茶歇与交流时间
14:30 Tea Break & Networking
15:00 圆桌讨论:ADC商业化之路:效率、成本与合规性如何动态平衡?上市后变更的监管弹性如何界定?
15:00 Panel Discussion: The Path to ADC Commercialization: How to Dynamically Balance Efficiency, Cost, and Compliance? How to Define the Regulatory Flexibility for Post-approval Changes?
议题包括:
· 生产原物料、检验方法及试剂变更时的转化策略;
· ADC复杂度提升及成本控制关键点(双抗、双Payload、多Payload、复杂Linker等);
· 成本与合规的平衡方法。
Moderator: Yuejun Xie, VP CMC Product Development, ACROBiosystems
主持:谢岳峻,CMC 产品开发副总裁,百普赛斯
Long Zhang, Co-founder, MindRank AI
张龙,联合创始人,德睿智药
Yun Zhou, Director of Conjugation & Formulation Production, TOT Biopharm
周昀,偶联及制剂生产总监,东曜药业
Shengnan Cai, CMC Statistics Lead, BeiGene
蔡圣楠,CMC统计负责人,百济神州
15:45 大会结束致辞
15:45 Closing Remarks
分论坛6 干细胞产品开发与CMC管理 9月4-5日
Forum 6: Stem Cell Product Development & CMC Management
4-5th September
大会主席:
Forum Chairs
Baozhu Yuan, Founder / Professor, Zhiding Bio / Tongji University
袁宝珠,创始人 / 教授,质鼎生物 / 同济大学
Changfeng Zhang, Quality & Registration Director, SPH Biotherapy (Shanghai Pharmaceutical Group)
张长风,质量与注册总监,上药集团生物治疗
13:30 干细胞治疗IND申报中CMC的管理
13:30 CMC Management in IND Application for Stem Cell Therapy
Changfeng Zhang, Quality & Registration Director, SPH Biotherapy
张长风,质量与注册总监,上药集团生物治疗
14:15 干细胞细胞株的筛选及细胞库建立研究
14:15 Research on Stem Cell Line Screening and Cell Bank Establishment
Wenwen Jia, Deputy Director / GMP Lab Director, National Stem Cell Transformation Resource Bank / Stem Cell Base of Oriental Hospital
贾文文,副主任/GMP实验室主任,国家干细胞转化资源库/东方医院干细胞基地
14:45 干细胞疗法注册和临床出海考虑点
14:45 Considerations for Stem Cell Therapy Registration and Global Expansion
Lijuan Chen, Senior Vice President, IQVIA
陈丽娟,sVP,IQVIA
15:15 干细胞治疗产品国产培养基的突围之路:对标进口方案及案例分享
15:15 Breakthrough Path for Domestic Culture Media for Stem Cell Therapy: Benchmarking Imported Solutions and Case Sharing
Xiaojian Zhao, Foreign Academician of the European Academy of Natural Sciences, Chairman & CEO, Bioinnovat
赵晓剑,欧洲自然科学院院士,董事长兼CEO,百因诺生物
15:45 茶歇与交流时间
15:45 Tea Break & Networking
16:15 干细胞产品自动化设备国产破局者:优势与案例分享
16:15 Domestic Pioneer in Stem Cell Product Automation Equipment: Advantages and Case Sharing
Linglong Zou, Chairman/CEO, Convalife Biotech
邹灵龙,董事长/CEO,康维讯生物
16:45 AI支持干细胞创新药临床开发和监管决策
16:45 AI Supporting Clinical Development and Regulatory Decision-making for Innovative Stem Cell Drugs
Jueyu Xia, Deputy General Manager, Jiangsu Tuohong Kangheng Pharmaceutical Co., Ltd.
夏珏妤,副总经理,江苏拓弘康恒医药有限公司
17:15 圆桌讨论:干细胞和干细胞外泌体成药(ATMP)的机遇,挑战和趋势
17:15 Panel Discussion: Opportunities, Challenges, and Trends in Stem Cell and Stem Cell Exosome Drug Development (ATMP)
议题包括:
· CDE创新细胞治疗药新分类征询意见带来的机会及国内外影响;
· 干细胞外泌体及干细胞药物的稳定性、异质性、规模化生产、质量保障等挑战;
· 国内外发展趋势探讨。
Moderator: Xiaojian Zhao, Foreign Academician of EANS, Chairman & CEO, Bioinnovat
主持:赵晓剑,欧洲自然科学院院士,董事长兼CEO,百因诺生物
Chunming Rao, Former Director, Recombinant Drug Dept., NIFDC
饶春明,生检所重组药物室原主任,中国食品药品检定研究院
Linglong Zou, Chairman/CEO, Convalife Biotech
邹灵龙,董事长/CEO,康维讯生物
Lijuan Chen, SVP, IQVIA
陈丽娟,sVP,IQVIA
18:00 第一天大会结束
18:00 Day 1 Conference Concludes
Day 2
第二天
09:00 诱导多能干细胞(iPSC)的快速高效制备及其衍生疫苗产品的设计与开发
09:00 Rapid and Efficient Preparation of Induced Pluripotent Stem Cells (iPSC) and Design and Development of Derived Vaccine Products
Fei Zhu, Associate Professor, XJTLU Wisdom Lake Academy of Pharmacy; Specially Appointed Expert Advisor, ISCO (Initiative on Stem Cells and Organoids)
朱飞,副教授,西浦慧湖药学院,干细胞与类器官创新计划组(ISCO)特聘专家顾问
09:45 iPSC技术突破与CMC工艺开发挑战
09:45 iPSC Technology Breakthroughs and CMC Process Development Challenges
Ying Zhang, Co-founder & CTO, CellOrigin (Zhong Sheng Su Yuan) Biotech Co., Ltd.
张颖,联合创始人&CTO,中盛溯源生物科技有限公司
10:30 茶歇与交流时间
10:30 Tea Break & Networking
11:00 iPSC衍生神经细胞产品自动化工艺及特殊质控方法开发
11:00 Development of Automated Processes and Special QC Methods for iPSC-derived Neural Cell Products
Anxin Wang, Head of R&D Center, Hopstem (HuoDe) Biotechnology
王安欣,研发中心负责人,霍德生物
11:30 干细胞外泌体监管现状及要求
11:30 Regulatory Status and Requirements for Stem Cell Exosomes
Baozhu Yuan, Founder / Professor, Zhiding Bio / Tongji University
袁宝珠,创始人 / 教授,质鼎生物 / 同济大学
12:00 午餐交流时间
12:00 Lunch & Networking
13:30 治疗急性缺血性卒中MSC新药的工艺开发与优化
13:30 Process Development and Optimization of a New MSC Drug for Acute Ischemic Stroke
Tian Li, R&D Director, Ingenious (Yinguan) Biotech
李田,研发总监,茵冠生物
14:00 诱导多能干细胞(iPSC)衍生细胞产品工艺开发与量产化
14:00 Process Development and Mass Production of Induced Pluripotent Stem Cell (iPSC)-derived Cell Products
Junfeng Wu, SVP CMC, Yuece Biotechnology (Ucyte)
吴军峰,CMC SVP,跃赛生物
14:30 茶歇与交流时间
14:30 Tea Break & Networking
15:00 圆桌讨论:破局干细胞疗法临床转化三问:早研、质量、工艺如何突破疗效天花板?
15:00 Panel Discussion: Breaking Through the Clinical Translation Bottleneck of Stem Cell Therapy: Three Questions on Early Research, Quality, and Process to Break the Efficacy Ceiling?
议题包括:
· 源头创新之问:如何通过早期研究设计出"更聪明、更持久"的下一代干细胞药物?
· 生产工艺之问:如何实现从"手工坊"到"工业4.0"的飞跃,保障细胞药物的可放大性、一致性及经济性?
· 质量体系之问:如何建立以"疗效"为导向的质量标准和放行策略,为临床疗效提供坚实保障?
Moderator: Yang Gao, Founder & CEO, Beijing Fange Technology Co., Ltd.
主持:高杨,创始人&CEO,北京凡歌科技有限公司
Baozhu Yuan, Founder, Zhiding Bio
袁宝珠,创始人,质鼎生物
Junfeng Wu, SVP CMC, Ucyte
吴军峰,CMC SVP,跃赛生物
Anxin Wang, Head of R&D Center, Hopstem Biotechnology
王安欣,研发中心负责人,霍德生物
15:45 大会结束致辞
15:45 Closing Remarks


< PAST · 往期回顾 >

▲ BioCMC 2023 第七届中国生物药CMC国际峰会

▲ BioAQ 2025 第三届中国生物药分析与质量峰会及ADC工艺与生产论坛

▲ BioAQ 2024 第二届中国生物药分析与质量峰会
百世传媒2013年起步于制药行业,立足感恩全力为用户满意深耕11年,专注于构建医药行业各细分领域专业技术论坛和峰会,促进行业经验、技术与人才交流,助力医药用户与合作伙伴从优秀走向卓越,携手建构中国医药影响力。
10年来百世传媒全力以赴为用户创造价值,已建构有口皆碑的品牌传媒服务: 十年经典峰会|CIS Asia化药峰会、生物药CMC峰会|BioCMC、百世药学院、百世药方舟、品牌研究院等。
百世传媒作为专业影响力的建构者,我们的品牌传播服务和质量在制药行业内有口碑,让合作伙伴信赖,我们秉承为健康铺路 让生命绽放的使命,致力于成为助力医药创新的最好媒介—让生命健康丰盈。
Luke Xia 手机:16628567478
Nina Zhu 手机:18317141348




百世药学院 | 医药领袖 | 药方舟 | 药博乐
扫码关注
带你发现更多精彩~